 Ammonia is a chemical compound of nitrogen and hydrogen with the formula NH3. It is a colorless gas that is about one half as dense as air at ordinary temperatures and pressures. It has a characteristic pungent, penetrating odor. Ammonia has also been called alkaline air and volatile alkali.
Ammonia is a chemical compound of nitrogen and hydrogen with the formula NH3. It is a colorless gas that is about one half as dense as air at ordinary temperatures and pressures. It has a characteristic pungent, penetrating odor. Ammonia has also been called alkaline air and volatile alkali.
Ammonia
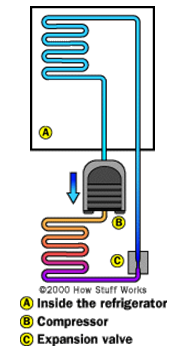
As a refrigerant, ammonia has four major advantages over CFCs and HCFCs:
• An ammonia-based refrigeration systems costs 10-20% less to build than one that uses CFCs because narrower-diameter piping can be used.
• Ammonia is a 3-10% more efficient refrigerant than CFCs, so an ammonia-based system requires less electricity, resulting in lower operating costs.
• Ammonia is safe for the environment, with an Ozone Depletion Potential (ODP) rating of 0 and a Global Warming Potential (GWP) rating of 0.
• Ammonia is substantially less expensive than CFCs or HCFCs
There are two key disadvantages to using ammonia as a refrigerant:
• It is not compatible with copper, so it cannot be used in any system with copper pipes.
• Ammonia is poisonous in high concentrations.
Two factors, however, mitigate this risk:
• Ammonia’s distinctive smell is detectable at concentrations well below those considered to be dangerous.
• Ammonia is lighter than air, so if any does leak, it will rise and dissipate in the atmosphere.
Other uses:
- Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers.
- Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceuticals.
- Ammonia solutions are used to clean, bleach, and deodorize; to etch aluminum; to saponify (hydolyze) oils and fats; and in chemical manufacture.
Ammonia Cylinders

Ammonia Drums

